2023 will be a pivotal year for biosimilar success or failure. Health care expert Fred Goldstein sits down with Stanton Mehr of Biosimilars Review & Report, to discuss what lies in store for biosimilars and AMCP’s biosimilar initiatives this year.
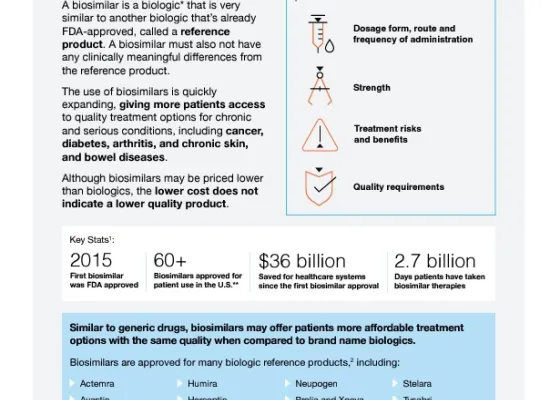
Biosimilars are biologic medicines that are highly similar to already approved reference products, offering comparable safety, efficacy, and quality at a reduced cost. Our resources provide information on the use, benefits, and regulatory guidelines of biosimilars to help healthcare professionals improve patient access and manage treatment costs effectively. To learn more about biosimilars and biologics visit AMCP's Biologics and Biosimilars Collective Intelligence Consortium (BBCIC) at bbcic.org.