USP and FDA Infographic on Biosimilars
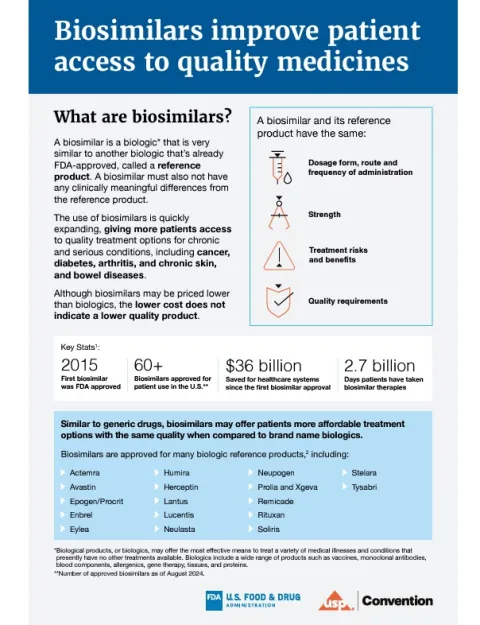
The use of biosimilars is quickly expanding due to their ability to treat diseases and help patients with chronic conditions that have previously lacked accessible treatment options, including cancer, diabetes, arthritis, and chronic skin and bowel diseases. Over the past nine years, more than 60 biosimilars have received US Food and Drug Administration (FDA) approval, saving healthcare systems over $36 billion. Ensuring availability of biosimilars can help improve patient access to life-changing, quality medicines. The United States Pharmacopeia (USP) and the FDA recently updated an educational resource on biosimilars to help facilitate conversations between healthcare practitioners and patients who can benefit from more accessible treatment options.
The updated resource highlights similarities between biosimilars and biologics, their FDA-approved reference products, such as the route of administration, dosage form, strength, quality and effectiveness. Just like biologics, biosimilars are rigorously tested by manufacturers following the same robust quality assessments, meaning that there are no clinically meaningful differences between a biologic and biosimilar for treatment of the same condition.
For additional information on biosimilars:
Featured News & Resources
See Full CalendarAward Applications Open
AMCP eLearning Day: Nexus Encore
AMCP 2026 Registration Opens
Upcoming Events
AMCP offers a wide variety of educational opportunities, from events and webinars to online training.