
Featured Legislative & Regulatory Positions
See all Positions
AMCP supports the implementation and expanded use of health information technology (HIT), including electronic health records and electronic prescribing.

The Academy of Managed Care Pharmacy (AMCP) supports an abbreviated licensure pathway for the approval of biosimilar biologic drug therapies.
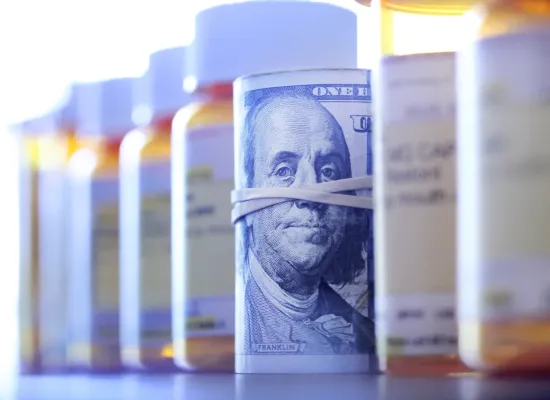
AMCP opposes government regulation of drug pricing because of the potential for unintended consequences and supports the elimination of barriers to competition to bring down the high cost of prescription drugs.
Featured Resources
See More Resources







Regulatory Updates
Letters, Statements, and Analysis
See All Letters, Statements, & Analysis
H.R. 1, or the Big Beautiful Bill, has brought substantial change to the managed care pharmacy landscape, particularly for managed care organizations (MCOs) with managed Medicaid lines of business.

HealthyWomen, members of its HPV Coalition and additional stakeholders are writing the Advisory Committee on Immunization Practices (ACIP) regarding the upcoming June ACIP meeting June 25-27, 2025, docket number - CDC-2005-0024.

Today, Senator Josh Hawley (R-MO) introduced legislation that would rollback some of the impending cuts to federal Medicaid funding as mandated by H.R. 1.









