RWE Standards Manuscript
The AMCP Research Institute has published a study in the Journal of Managed Care and Specialty Pharmacy (JMCP) entitled “AMCP real-world evidence standards: Overcoming barriers to using real-world evidence in US payer decision-making.” This paper describes the RWE standards developed as part of the AMCP RWE Initiative.
AMCP is working with health care stakeholders to overcome challenges and enhance the integration of Real-World Evidence (RWE) into health care decision-making.
This initiative brings together individuals widely recognized as experts in RWE due to their contributions to the field, their leadership in research, or their influence in shaping RWE methodologies and policies and organizations at the forefront of advancing the use of RWE in health care, including:
- Health plans
- Integrated Delivery Networks (IDN)
- Pharmacy Benefit Managers (PBM)
- RWE experts
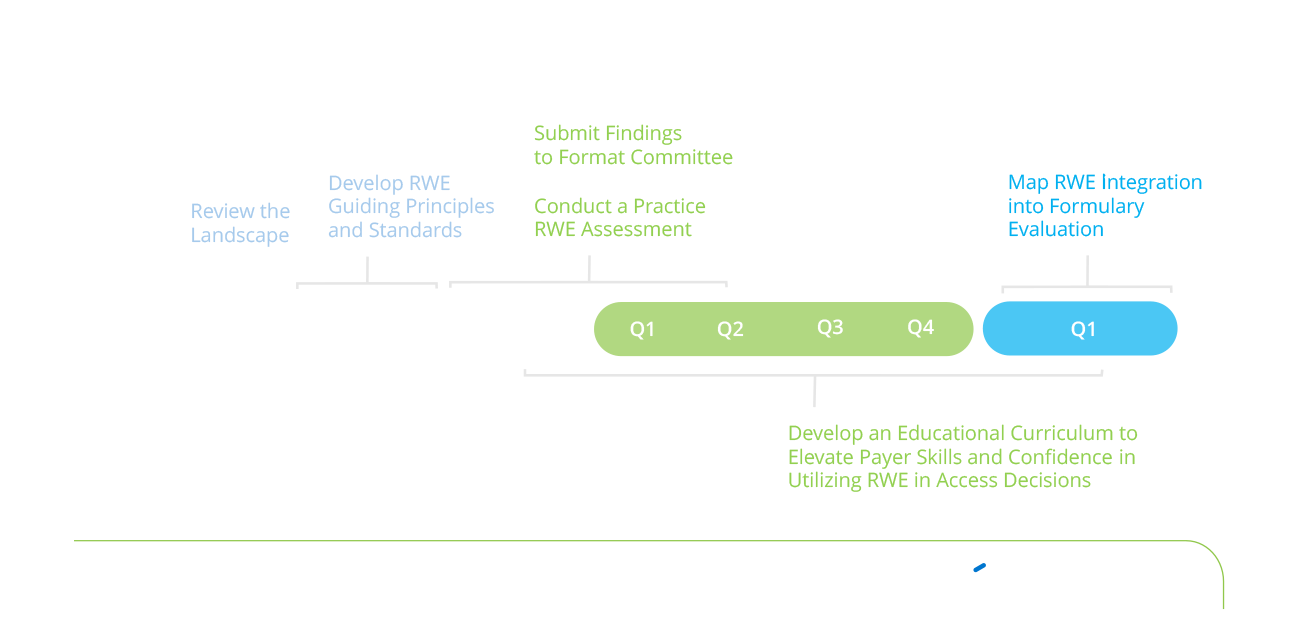
Together, we will provide essential thought leadership, create RWE standards for managed care, integrate these standards into the AMCP format, and provide education and practical application on how to use the RWE standards in evaluating formulary access.
The work will span two years with multiple touch points for health care stakeholders. If you want to learn more or interested in the project, let us know.
Key 2024 Deliverables:
- Payer RWE Engagement Survey: A targeted survey was conducted to investigate the underlying reasons for payers' hesitancy in embracing RWE. The survey also identified essential prerequisites for the consistent and effective use of RWE in formulary decision-making.
- Literature Research: A comprehensive review of relevant literature was carried out to gather insights and inform the development of the Standards for RWE utilization.
- Payer and Pharmaceutical Focus Groups: Two focus groups, comprising payers and pharmaceutical representatives, were held to refine terminology, validate survey findings, and provide additional insights. These discussions explored how the proposed Standards could be operationalized within payer formulary decision-making processes.
- Partnership Forum: In November 2024, the findings from this initiative were presented at a Partnership Forum, where AMCP will invite RWE thought leaders and relevant organizations to provide feedback. Insights from this forum will be integrated into the final RWE Standards. These will be published in the Journal of Managed Care & Specialty Pharmacy and submitted to the AMCP Format Committee for consideration to add to the AMCP Dossier.
- View Video.
- Read Blog: ICYMI: Recapping AMCP's Real-World Evidence Partnership Forum
- Read Blog: AMCP Real-World Evidence (RWE) Partnership Forum: A Student’s Perspective


2025: Expanding the Reach and Impact of RWE
In 2025, AMCP will enter the next phase, focusing on expanding the awareness and implementation of the RWE Standards across the healthcare ecosystem. A dedicated strategy will be implemented to equip payers with the knowledge, tools, and confidence necessary to effectively leverage RWE.Highlights:
- Best Practices for Enhanced Adoption: Along with the Standards, AMCP will formulate and disseminate industry best practices for evaluating RWE. This will ensure alignment between pharmaceutical companies and payers in understanding and applying RWE.
- Market Education: AMCP will launch targeted educational programs designed to increase the understanding of RWE among key stakeholders, including payers, pharmaceutical companies, and healthcare providers. These programs will address the practical aspects of incorporating RWE into health plans and access decisions along with pharmaceutical RWE study design.
- RWE Repository: Research conducted in 2024 revealed a significant need for an FDA-compliant RWE repository. Such a repository will provide a centralized, accessible platform where payers can easily access high-quality, relevant RWE studies, fostering transparency and improving efficiency in evidence-based decision-making.
Past Events & Resources
AMCP 2025: Unlocking the Value of Real-World Evidence (RWE): Insights for Payers and Manufacturers
March 31-April 3, 2025, Houston, Texas
Take Course
Webinar: Bridging the Gap: Development of Real-World Evidence (RWE) Standards to Facilitate Communication Between Manufacturers and Payers through the AMCP RWE Initiative
Partnership Forum – Closing the Gap in Real-World Evidence Use: A Collaborative Approach for Health Care Transformation
November 12-13, 2024
This Partnership Forum will convene multi-stakeholder experts to develop resources that will address barriers to RWE use in formulary decision-making through workshop and collaborative activities that will result in a set of RWE standards and tools to facilitate stakeholder communication and streamline the use of RWE.
Other RWE Resources